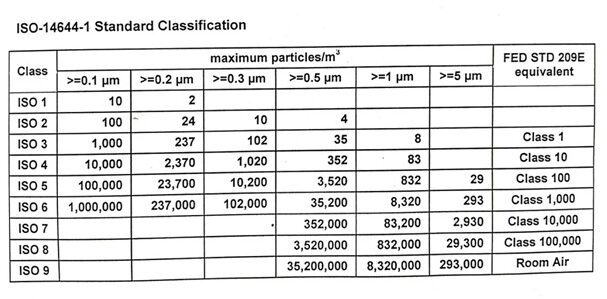
Cleanroom injection molding is particularly relevant in the manufacturing of medical devices, which are categorized based on the level of risk they pose to patients and users:
Class I Devices: These devices have minimal contact with patients and a low impact on overall health. Examples include electric toothbrushes, tongue depressors, and bandages. Cleanroom injection molding may not be required for such devices due to their minimal risk level.
Class II Devices: Class II devices present a higher risk as they come into sustained contact with patients. Examples include catheters, blood pressure cuffs, and syringes. Cleanroom injection molding in an ISO 8 environment is often suitable for these devices, ensuring sterility and regulatory compliance.
Class III Devices: Class III devices, such as pacemakers and breast implants, carry the highest risk and complexity. These devices typically require the most stringent regulatory controls and are assembled and molded in cleanrooms, aiming to minimize contamination from airborne particles.