Clean room injection molding
Clean room injection molding is a critical manufacturing process that ensures precision, quality, and cleanliness in the production of components for various industries. We will delve into the concept of clean room injection molding, highlight its benefits, and examine real-world case studies that demonstrate its effectiveness in achieving superior results. Discover how clean room injection molding has transformed manufacturing processes across different sectors.

Benefits of Clean Room Injection Molding
Clean room injection molding offers a range of advantages that contribute to enhanced precision and quality
Contamination Control: The controlled environment of a clean room minimizes the risk of contamination, ensuring the production of components that meet the highest cleanliness requirements. This is crucial in industries such as medical and healthcare, where sterility is essential.
Improved Quality Assurance: Clean room conditions significantly reduce the potential for defects caused by environmental contaminants. The controlled environment allows for consistent manufacturing processes, resulting in tighter tolerances, improved part-to-part consistency, and reduced rework or rejection rates.
Regulatory Compliance: Industries with stringent regulatory standards, such as medical, pharmaceutical, and aerospace, benefit from clean room injection molding. Compliance with regulations ensures that components meet the necessary certifications and approvals
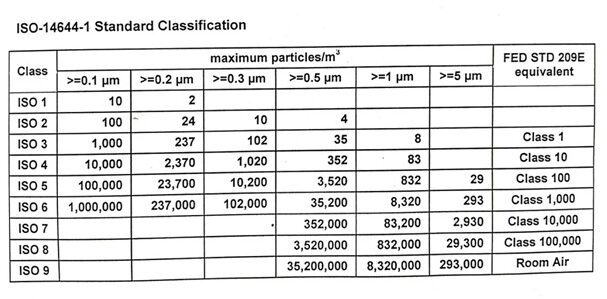
ISO 14644-1 Standards
ISO stands for International Standard Organization which comprises various organizations from different countries. These countries work together to develop and publish standards that are internationally valid and thus, need to be adhered to across borders.
Since 2001, the cleanroom classification of classes ISO 1 – ISO 9 of ISO 14644-1 has been in effect. ISO 14644-1 defines the degree of purity of the air by determining limit values. ISO classification is upon the basis of particle concentration per m³. The highest purity in ISO 14644-1 is in ISO class 1, whereas the lowest is classified as ISO class 9. ISO Class 9 is also categorized as Room air. The table below depicts the ISO 14644-1 Cleanroom
Class 7 Cleanroom
A Class 7 Cleanroom serves as a dedicated, enclosed space for injection molding, separated from the rest of the facility by solid walls. Air circulates continuously within the room, undergoing purification and monitoring to comply with ISO 14644-1 standards. The particulate limits per cubic meter are:
- Particulates ≥ 0.5 µm: Up to 352,000 particles
- Particulates ≥ 1.0 µm: Up to 83,200 particles
- Particulates ≥ 5.0 µm: Up to 2,930 particles
Cleanroom Injection Molding Requirements
Key differences separate cleanroom molding from standard manufacturing floors:
- Positive Air Flow
Both Class 7 and 8 cleanrooms maintain controlled environments using positive air flow to regulate particulate levels. - Protective Gear
Engineers and operators entering a Class 7 Cleanroom must wear full-body gowns, shoe covers, and hair nets. Class 8 environments often relax these standards slightly. - Electric Machines
Cleanroom operations favor electric over hydraulic machines to minimize airborne particle generation. - Packaging Restrictions
Cleanroom rules restrict the use of particle-generating materials like untreated corrugated cardboard. Instead, manufacturers use coated cardboard or plastic packaging to preserve cleanliness.
By following these practices, manufacturers ensure product integrity, especially in applications requiring sterility and precision.
Post-Molding Operations in Cleanrooms
Cleanroom injection molding plays a vital role in producing medical devices, which fall into three categories based on patient risk:
- Class I Devices
These low-risk devices, such as bandages and tongue depressors, rarely require cleanroom molding. - Class II Devices
Devices like catheters and syringes come into sustained contact with patients. Manufacturers often produce these in Class 8 Cleanrooms to meet sterility standards. - Class III Devices
Pacemakers and breast implants involve the highest patient risk and require strict control. Production typically occurs in Class 7 Cleanrooms to prevent contamination and meet complex regulatory demands.
Real-World Case Studies
Case Study 1: Medical Device Manufacturing
A medical device company needed sterile, high-precision plastic components for surgical instruments. They implemented Class 7 Cleanroom injection molding, eliminating contamination risks and achieving tight tolerances and repeatable results. The sterile components helped the company meet strict regulatory requirements and deliver exceptional product performance.
Case Study 2: Pharmaceutical Packaging
A pharmaceutical manufacturer required sterile plastic containers for sensitive medications. Using cleanroom injection molding ensured container sterility and dimensional consistency. This controlled process maintained product safety and supported reliable closure mechanisms, improving usability and protection.
Case Study 3: Biotechnology Research
A biotech firm needed microfluidic chips with complex internal structures for lab research. Cleanroom molding prevented contamination, ensured accurate dimensions, and delivered consistent chip quality. As a result, the company improved its research reliability and enabled advanced testing.
Categories
Recent Posts
Contact Us
Error: Contact form not found.





